
The first FDA approved non-mask therapy designed to treat CSA
The remedē® System is an implantable system that safely and effectively treats moderate to severe central sleep apnea (CSA) in adult patients.1
RESTORES a more normal breathing pattern by using the body’s own breathing system.
TAILORS therapy to each patient through customized programming that attempts to mimic natural breathing while asleep.
RELIEVES patient compliance concerns by automatically delivering therapy each night.
ELIMINATES external equipment using a mask-free, implantable device that is placed under the skin int he upper chest area during a minimally invasive, outpatient procedure.
See how the remedē System is implanted
The Latest Advancement in remedē® System Technology
The remedē EL-X System is a next generation platform combining enhanced functionality with a patient-friendly design that simplifies the implant procedure and delivers long-lasting benefits to your adult patients with central sleep apnea.
Next Generation Platform
Extended longevity
Long-lasting battery life extended by 40% from previous platform1
Patient-friendly design
Small and thin; size decreased by 25% from previous model1 with rounded edges designed for comfort
Simplified Implant
Single lead, single port system
Delivers stimulation and senses respiration from a single lead, simplifying the implant experience

Next Generation remedē Reports
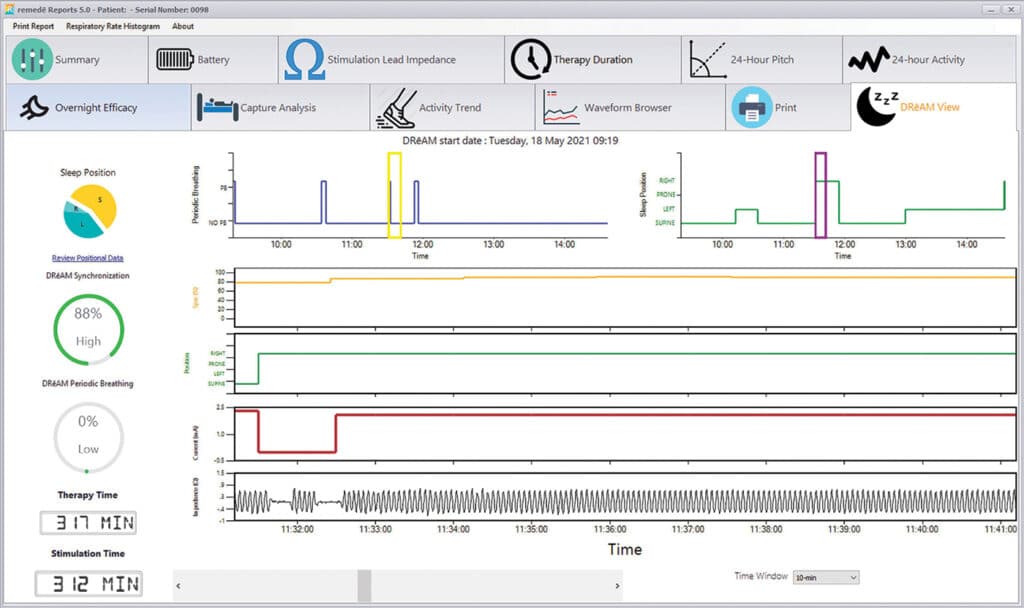
Enhanced with DRēAM View
remedē Reports software is designed to deliver insightful information for you to efficiently tailor therapy for your patients. The latest enhancement, DRēAM View, provides an enhanced set of full-night, detailed data detected by the device.
remedē EL System
Built on the same next-generation platform, the remedē EL is designed for compatibility with current IS-1 leads.


