
2025 American Academy of Sleep Medicine CSA treatment guidelines
Transvenous Phrenic Nerve Stimulation (TPNS) recommended as a treatment option in the 2025 AASM CSA guidelines1
In 2025, the American Academy of Sleep Medicine updated its guidelines for evidence-based treatment of central sleep apnea to include remedē System as a treatment option.
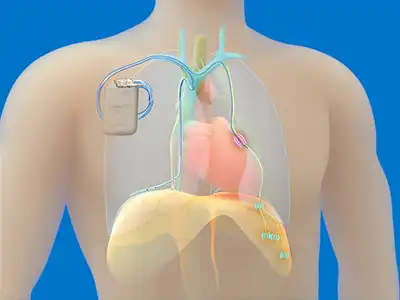
As cited in the updated guidelines, the period since the last AASM guidelines on CSA was notable for two important developments featured in these guidelines. “The first is the development of the TPNS, a fully implantable neurostimulator that became Food and Drug Administration (FDA) approved and commercially available (remedē®, Zoll) in 2017″
- Badr MS et al. Treatment of central sleep apnea in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2025 in press.
Summary of recommendations for adults with Central Sleep Apnea1
| Intervention | Strenth of recommendation | Critical Outcomes Meeting the Clinical Significance Threshold2 | ||
|---|---|---|---|---|
| Excessive sleepiness | Disease severity | Cardiovascular Disease | ||
| TPNS3 | Conditional for | ✔ | ✔ | ✔ |
| CPAP4 | Conditional for | ✔ | ||
| BPAP w/ backup rate5 | Conditional for | ✔ | ✔ | ✔ |
| BPAP w/o backup rate4 | Conditional against | ✔ | ✔ | |
| ASV4 | Conditional for | ✔ | ||
| Low-flow oxygen6 | Conditional for | ✔ | ||
| Acetazolamide4 | Conditional for | ✔ | ✔ | |
- Does not include central sleep apnea due to high altitude
- Excluded hospitalizations, sleep quality, and mortality, for which no intervention met the Clinical Significance Threshold or could not be assessed
- Primary CSA and CSA due to heart failure
- Primary CSA, CSA due to HF, CSA due to medication or substance use, treatment-emergent CSA, and CSA due to a medical condition or disorder
- Primary CSA, CSA due to medication or substance use, treatment-emergent CSA, and CSA due to a medical condition or disorder
- CSA due to heart failure
The conditional recommendation suggests most patients in the appropriate population should be offered TPNS as an option.
It’s time to ACT on the CSA guidelines: help your patients learn about TPNS
A
TPNS was added into the new guidelines
“The AASM suggest using transvenous phrenic nerve stimulation (TPNS) over no TPNS in adults with CSA due to the following etiologies: primary CSA and CSA due to heart failure therapies.”
C
The conditional recommendation means most eligible patients should be offered TPNS
“Most patients should be offered the suggested course of action” (Table 1—Implications of strong and conditional recommendations)
T
Try alternatives when central events or symptoms persist
“Persistence of central respiratory events should prompt re-evaluation of the underlying risk factors and consideration of alternative treatment options.”
“Clinicians must prioritize optimizing therapy for the conditions contributing to central apneas and improving patient-reported outcomes rather than solely focusing on eliminating disordered breathing events”

Choosing remedē for your CSA patients
FDA-approved in 2017, the remedē System is the only transvenous phrenic nerve stimulator available to patients with CSA.
Additional resources

2025 AASM Guidelines
Read AASM’s latest recommendations for evidence-based treatment of CSA events
Guidelines takeaways
A convenient summary of AASM’s 2025 treatment recommendations

Who is the remedē System for?
Explore detailed information about patient selection
Interested in remedē for your patients?
If you want to offer remedē to your patients and/or become a remedē center, connect with one of our representatives.

