Resources to Support the Patient Pathway
Patient Selection for Phrenic Nerve Stimulation with remedē®
The remedē System is FDA approved for the treatment of moderate-to-severe CSA in adult patients1 with:
- No requirement to have tried and failed other therapies for CSA
- No upper limit of Apnea Hypopnea Index (AHI)
- No upper limit of patient Body Mass Index (BMI)
The remedē System is contraindicated for patients with an active infection.
Appropriate candidates for the remedē System may have events
other than central apneas noted during the sleep study
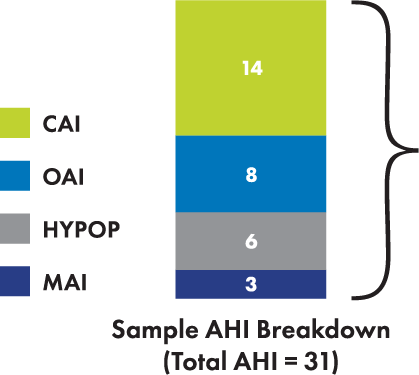
Patients for remedē therapy should have moderate-to-severe sleep apnea with AHI ≥ 15
In the remedē Pivotal Trial, patients had more central apneas than obstructive apneas2
Hypopneas may be central or obstructive in nature and do not exclude patients for remedē therapy
remedē Patient Selection Pathway Example
- Costanzo, et al. Lancet 2016; 388: 974–82.

